Focus View all

Important hub, for its strategic geographical position, to Europe, West Africa and the Atlantic route, Morocco is a country poor in natural resources and raw materials but with huge intangible resources such as art, history and culture. Rich in savoir fair, artisan tradition, skilled negotiators,......
Infomedix International 3 / 2025Products Looking for Distributors
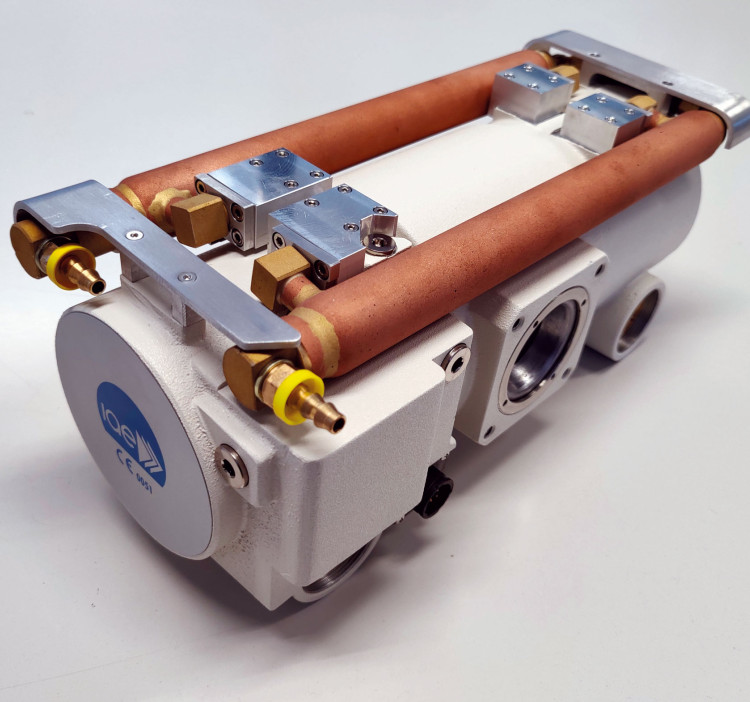
IAE SpA - New Housing C 25
IAE is a major role player in the international x-ray market as the only independent manufacturer in Europa of rotating anode tubes. With its wide product line of more than 100 insert/housing combinations, IAE is a strategic and reliable partner to the most important equipment manufacturers globally. A recently developed product is a rotating anode x-ray unit, suitable for C-arms; it features a compact design with miniature high voltage connectors and a single-piece cast aluminium body. A combination of 2 tubular oil-water heat exchangers on the housing body and a remote air-water heat exchanger ensures amazing heat dissipation. Visit us at RSNA 2025Hall 3, Booth 3961
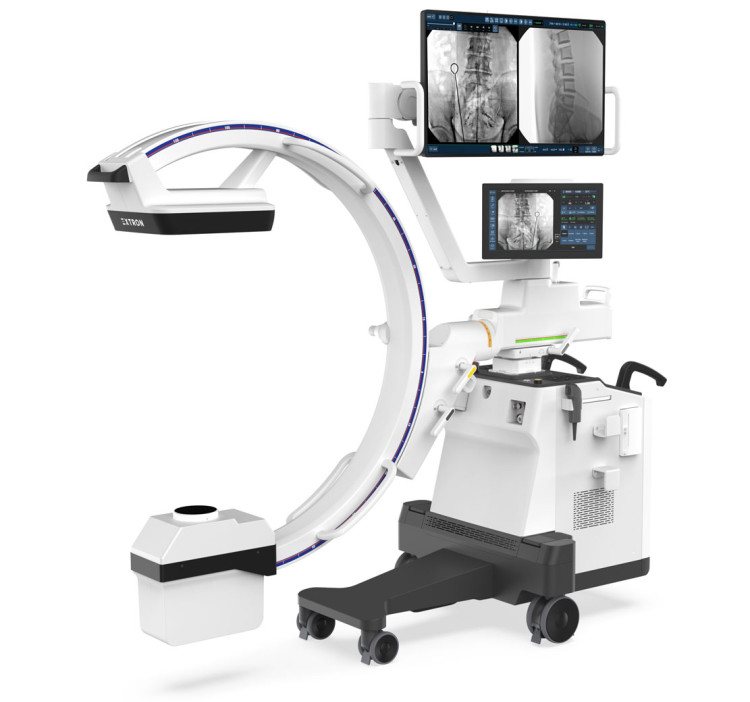
DRTECH - EXTRON 3: Proven Image Quality, Enhanced Safety and Efficiency
Introducing EXTRON 3, the latest addition to DRTECH’s premium low-dose C-arm series. Building upon the proven performance of the MDR-certified EXTRON, the new EXTRON 3 delivers the same outstanding TruDigital image quality with even greater accessibility. Designed for smaller surgical environments, EXTRON 3 combines compact mobility with advanced low-dose imaging, ensuring radiation safety for patients and clinicians. Its enhanced RNR (Real-time Noise Reduction) and AOD (AI Object Detection) technologies provide crystal-clear, stable images while minimizing artifacts and halation during procedures. Equipped with a 43” monitor, a lightweight yet stable and compact cart, and an intuitive 15.6” touch console, EXTRON 3 delivers effortless operation and an optimized workflow. With its balance of performance, efficiency, and cost-effectiveness, EXTRON 3 is the ideal choice for hospitals seeking a streamlined, next-generation mobile C-arm. Visit us at MEDICA 2025Hall 9, Booth D50

Forumed SL - Bacterial Viral filters for Spirometry
FORUMED bacterial viral filters eliminate any potential risk of cross-infection during pulmonary function tests. They are available with different connection sizes and fittings, making them suitable for most machines and pipes on the market. Pulmonary function tests are noninvasive tests that show the lungs healthy condition. The tests measure lung volume, capacity, rates of flow, and gas exchange. Compatible sizes with : MGC, Jaegger, MIR, Cosmed, Medisoft, Spiroconnect, Koko, Medgraphics, Sensormedics, Geratherm, Vitalograph Visit us at MEDICA 2025

J.D. Honigberg International Inc. - BOWMAN DISPENSERS. Organize – Contain - Comply
Bowman PPE Dispensing Solutions • Quality materials and fabrication • End user design collaboration • Unique solution driven designs • Good, better, and best cost options • Organization that improves compliance • Easy purchasing process • Custom design abilities • Made in USA Visit us at MEDICA 2025Booth 16-D11-1

IEC - Endoscope CCD Camera Repair Innovation
In an effort, to make a better and more functional product to serve the requests of our loyal customers, IEC has developed a new design for our small diameter endoscope CCD’ repairs. This new design significantly reduces the diameter of the wire harness creating more room for other parts. It gives the endoscope CCD’ the ability to angulate and move with less stress on the camera than ever before. We offer this service to endoscope repair companies and dealers worldwide. Please contact us: Innovative Endoscopy Components, LLC: 320 International Parkway, Fort Lauderdale, FL 33325, USA Tel: 954-217-8780, Fax: 954-217-8781

IEC - Premium Endoscope Repair Parts
Innovative Endoscopy Components, LLC has been the ISO13485/ISO9001 Certified vendor of choice to hundreds of endoscope service facilities and dealers worldwide, for over 23 years. Our product range and services are constantly growing with international demand. Rapid prototyping, optical assemblies, injection molding, and CNC machined parts are offered just like OEM endoscope and equipment labeling as well as CCD’ repair and multilingual repair training and consulting. Please contact us: Innovative Endoscopy Components, LLC: 320 International Parkway, Fort Lauderdale, FL 33325, USA Tel: 954-217-8780, Fax: 954-217-8781